What is electrophysiology in the context of neuroscience?
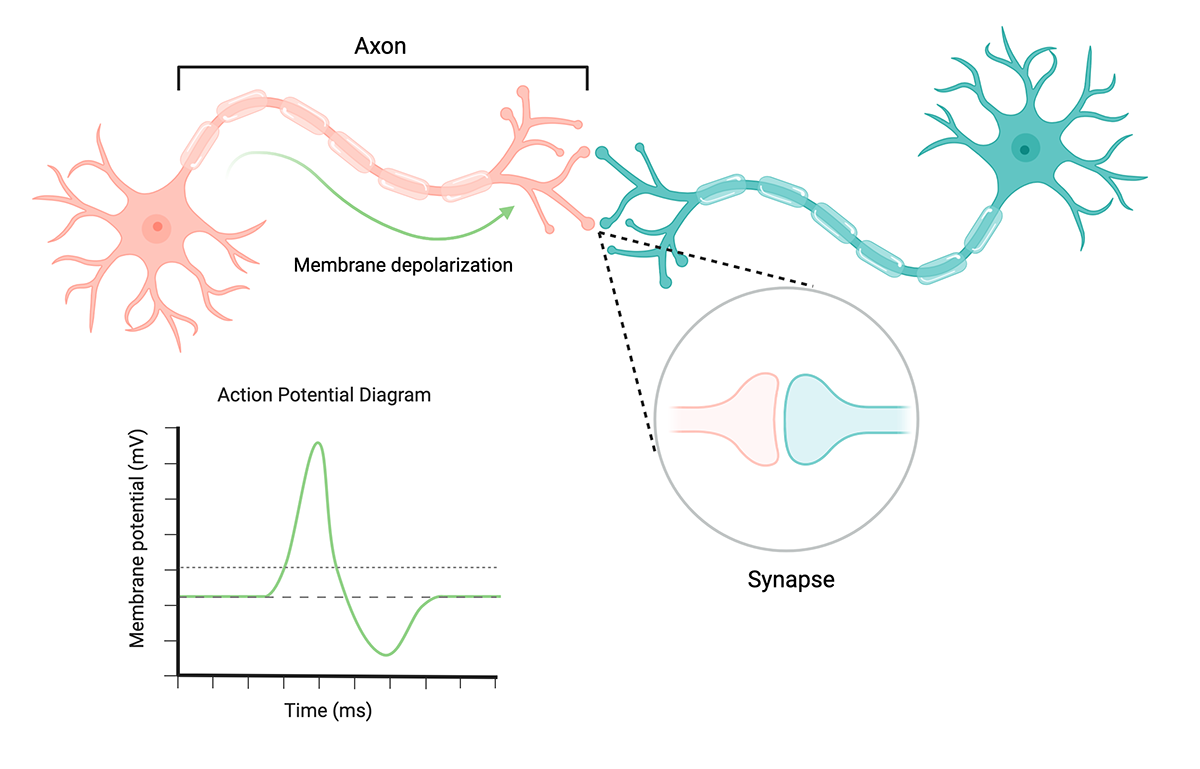
Electrophysiology is the study of the electrical properties of biological matter. Neurons possess unique electrophysiological properties that enable them to rapidly communicate with each other throughout the brain and peripheral nervous system. Electrical communication between neurons occurs by generating signals called action potentials that travel through specialized parts of cells (axons) and trigger neurotransmitter release across junctions between cells (synapses).
When neurotransmitters attach to receptors, they enable the flow of ions (like Na+ or K+) across membrane channels into the neuronal cell. A neuronal cell’s resting membrane potential (difference in charge between inside and outside of cell) is typically -70 mV. As ions flow into the cell, the membrane potential becomes less negative (depolarization). When the membrane potential reaches -50 mV, the cell generates an action potential, which propagates down the axon and triggers neurotransmitter release across the synapse, further triggering electrophysiological responses in another neuronal cell. These “spikes” in electrophysiological data are analyzed to deduce patterns in neural activity and communication between cells.
The basics of neuronal cell communication. Membrane depolarization reaches an action potential threshold of -50V, producing an action potential that propagates through the axon and triggers neurotransmitter release at the synapse. Created with BioRender.
Why is studying neural electrophysiology important?
Electrophysiological data are direct measurements of neural activity, enabling quantitative assessments of neural function and connectivity. These assessments can be used to investigate the basis of pathological changes in neural function, such as those that may occur in neurological disorders or nervous system injury. For example, researchers in one study monitored neural activity changes in co-cultures of neurons, astrocytes, and microglia that mimicked neuroinflammatory conditions to determine the impact of inflammation on neural function.
Neural electrophysiology is also crucial to drug development. Monitoring neural function changes in vitro is an important part of assessing the impact of drugs for neurological and psychiatric diseases before trials can make it to patients. With improving technology in induced pluripotent stem cells (iPSCs), researchers can now perform electrophysiological studies on neurons generated from patient-derived iPSCs to see how the drug may individually impact patients.
In addition to assessing a drug’s function, neural electrophysiology is also used to measure drug safety. In vitro electrophysiology enables preclinical neurotoxicity screening for drugs as well as other compounds with potentially hazardous effects on the nervous system, like environmental agents.
What tools are used to investigate neural electrophysiology?
Most approaches to measuring electrophysiology in cultured neuronal cells are derived from one of the following methods: patch-clamping, multi-electrode arrays (MEAs), or optical imaging (calcium or voltage).
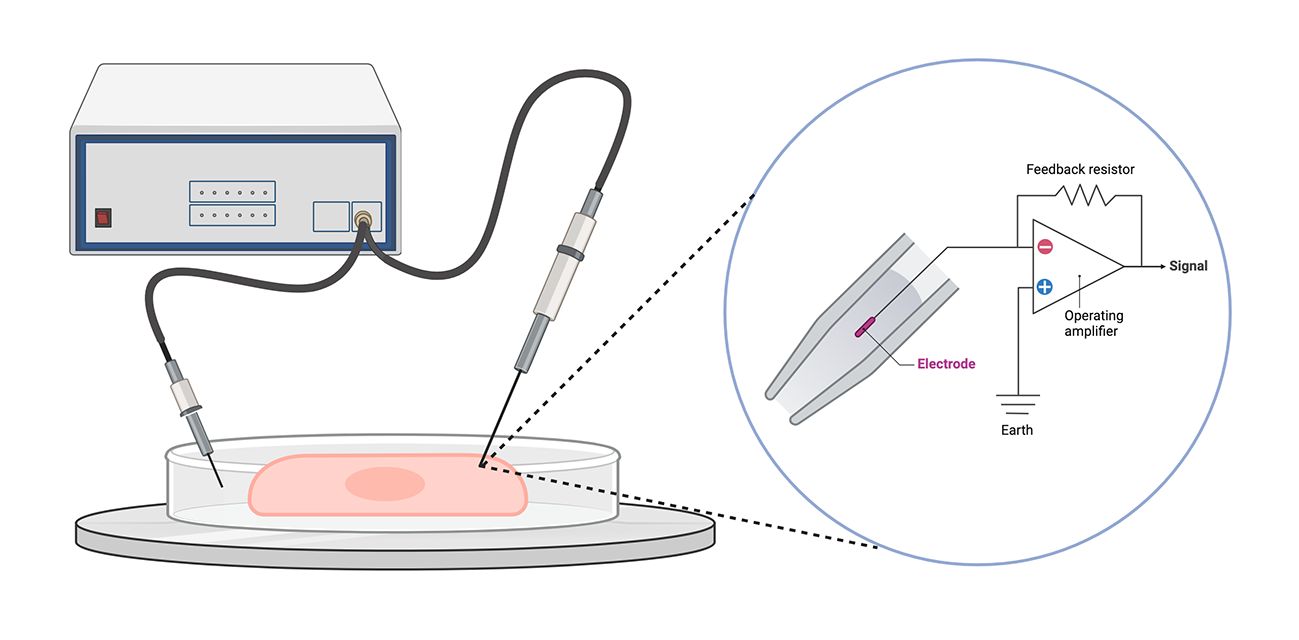
Patch-clamp is an electrophysiological technique commonly used to measure the ion-channel function, synaptic activity, and membrane potential of neurons. Patch-clamping consists of pressing the tip of a specialized pipette against a cell, then carefully applying suction to tightly seal the cell to the pipette tip. The pipette is connected to a circuit that feeds the electricity flowing from the cell into a converter that translates the current into a readable voltage.
While this technique offers high temporal and channel resolution (sub-millisecond and single channel, respectively), it is an invasive method that requires substantial technical proficiency and provides relatively low throughput. If the seal is not tight enough, current leakage can lead to inaccurate data. Incorrect patch-clamping can also damage cells. Advances in automated patch-clamp approaches are improving throughput and ease of use, but to a limited degree.
Schematic of a patch-clamp setup, with a close-up of the specialized pipette tip. Patching a single cell can be incredibly difficult and potentially destructive. Created with BioRender.
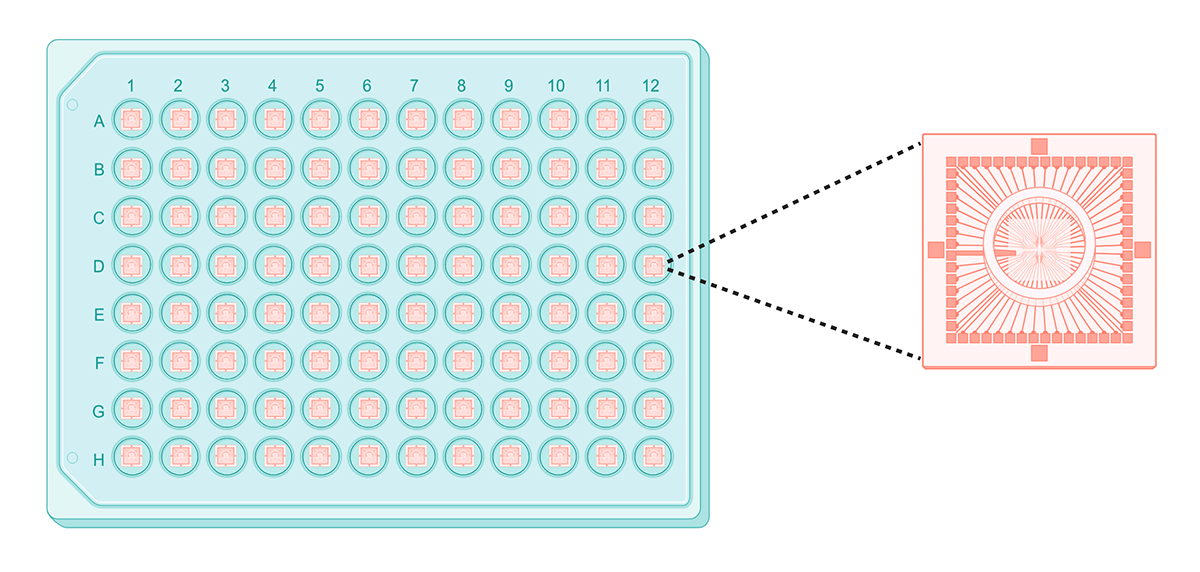
Multi-electrode arrays (MEAs) are another tool for measuring electrophysiological properties of cells. MEAs comprise specialized tissue culture plates with electrodes in each well. Cells grow on top of the electrodes, which measure their electrical activity over time.
Unlike patch-clamping, MEAs are a noninvasive technique that can measure neural activity across large populations of cells for a relatively long time. Additionally, integration of MEAs into multi-well microplates – such as 48- and 96-well plates – significantly improve their throughput compared to patch-clamping. However, many MEAs sacrifice spatial resolution for throughput. High-resolution data capture is typically restricted to small microplates, like 1-, 4, or 6-well formats. Higher-throughput MEAs have only a few electrodes per well, recording neuronal activity for populations of cells rather than individual neurons.
A typical multi-electrode array, with a close-up of electrodes in a single well. Created with BioRender.
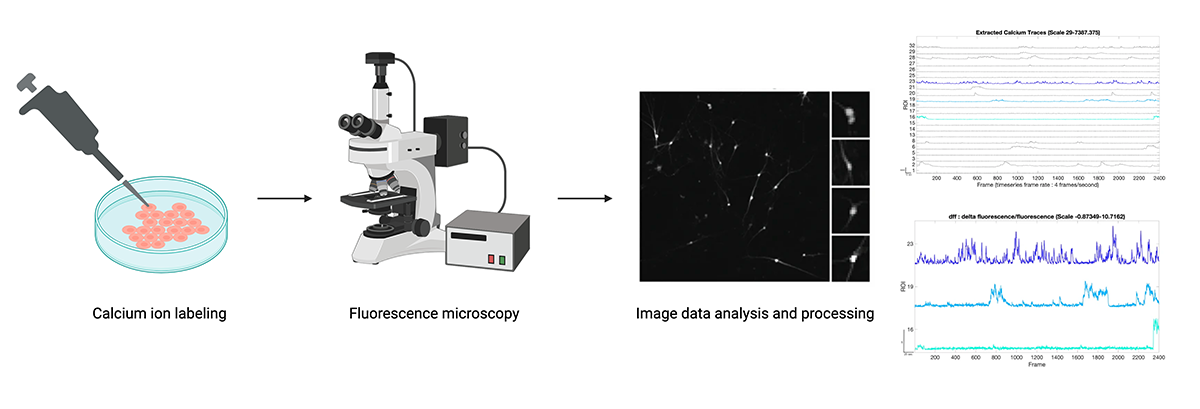
There are also optical imaging-based methods for measuring the electrophysiological activity of neuronal cells. Calcium imaging focuses on detecting changes in calcium ions, which play many critical functions in neuronal signaling. This is an indirect measurement technique, as microscopy images must be processed and converted into neural spike data. Voltage imaging, on the other hand, is a technique that directly measures neural electrophysiology by using voltage-sensitive dyes or transgenic cells that express voltage indicators to detect changes in electrical activity.
While these techniques can provide unique spatial insights into neural activity, they introduce new implementation challenges that limit the feasibility of obtaining high-throughout, longitudinal data. Optical imaging methods rely on optimizing fluorescent dyes or developing transgenic reporter cell lines, which are time- and labor-intensive processes. They also do not allow for long-term longitudinal measurements in vitro, only capturing a few snapshots of electrophysiological activity.
Simplified calcium imaging workflow. Image data must be translated into electrophysiological signals. Figures from “Image data analysis and processing” step from Tippani et al. (BMC Neuroscience 2022). Created with BioRender.
What are the limitations of these tools?
While traditional techniques based on patch-clamp technology generate data with high spatial and temporal resolution, they are difficult to execute, invasive and destructive to cells in a manner that does not allow for long-term monitoring, and low-throughput. Higher throughput, noninvasive tools like MEAs compromise resolution for scalability, with microplates above 24 wells only capable of monitoring neural activity at the population level. Meanwhile, optical imaging-based methods can involve complex protocols for label/dye optimization or transgenic cell line generation, and do not track changes in neural activity over long periods of time.
Regardless of their individual merits and limitations, all of these methods share the same major drawback: they only capture neural function, entirely missing cellular phenotype. Obtaining phenotypic data such as cell health, behavior, and morphology requires multiple assays – each with their own set of tools and data analysis challenges – on the same cells. These additional assays not only add time and effort to experimental pipelines, but also create additional opportunities for cells to die or become damaged.
How can we overcome these limitations?
Combining throughput, resolution, and longitudinal measurements is critical to garnering novel insights into neural function at scale. Integrating CMOS microelectrode arrays with 96-well microplates, the CytoTronics Pixel™ system is an ultra-high-density MEA capable of recording real-time data for thousands of wells at high resolution. Experiments can be run on the Primo single plate reader, or in the Octo 8-plate reader and benchtop incubator for increased throughput.
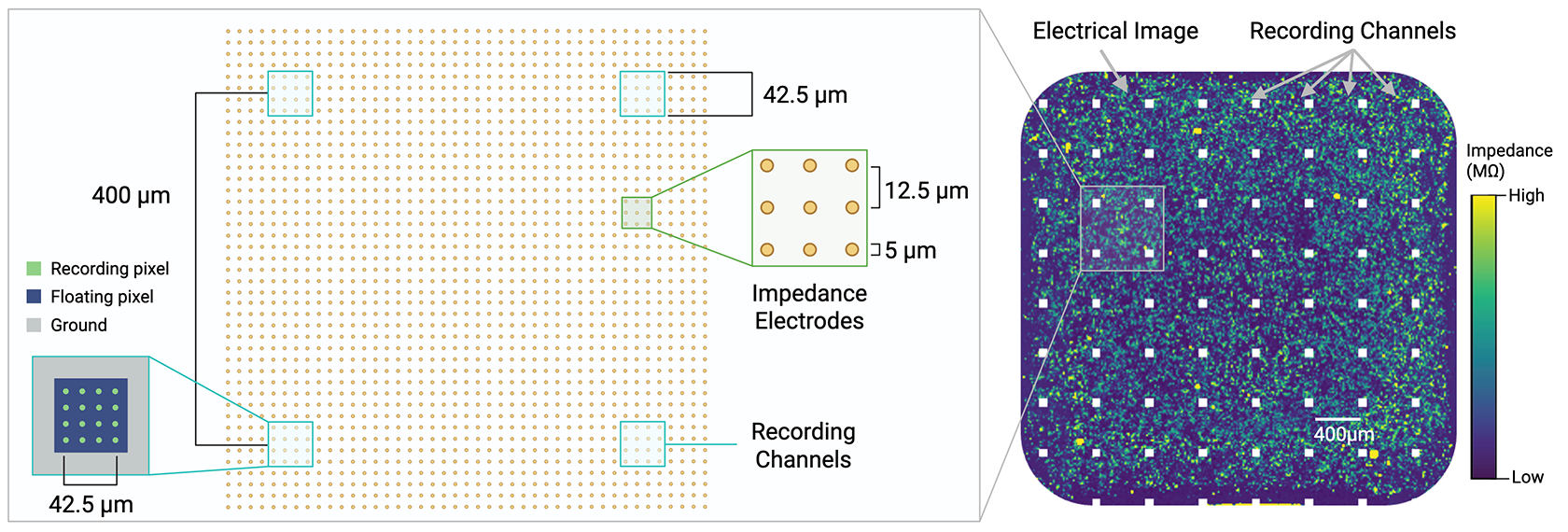
Recording channels are spaced every 400um within the microelectrode array. Impedance electrodes, with a resolution of 12.5um, generate an electrical image, while recording channels record neural firing.
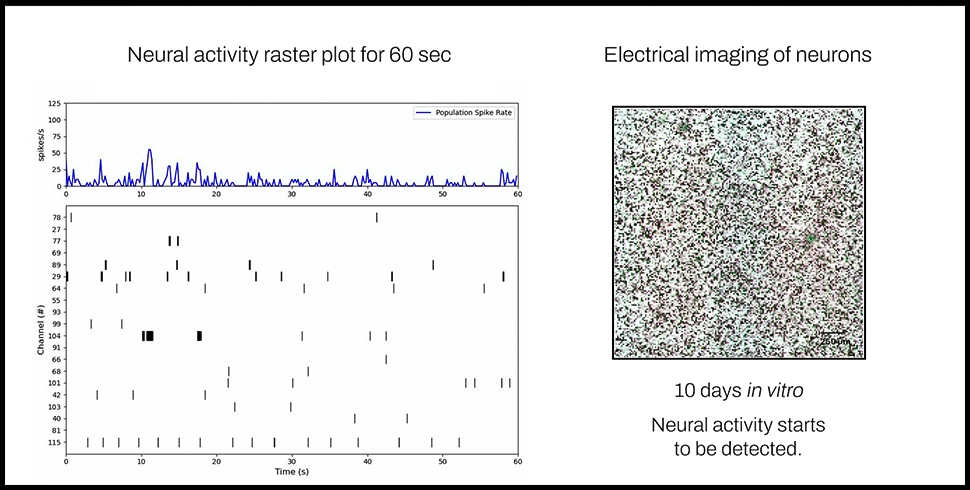
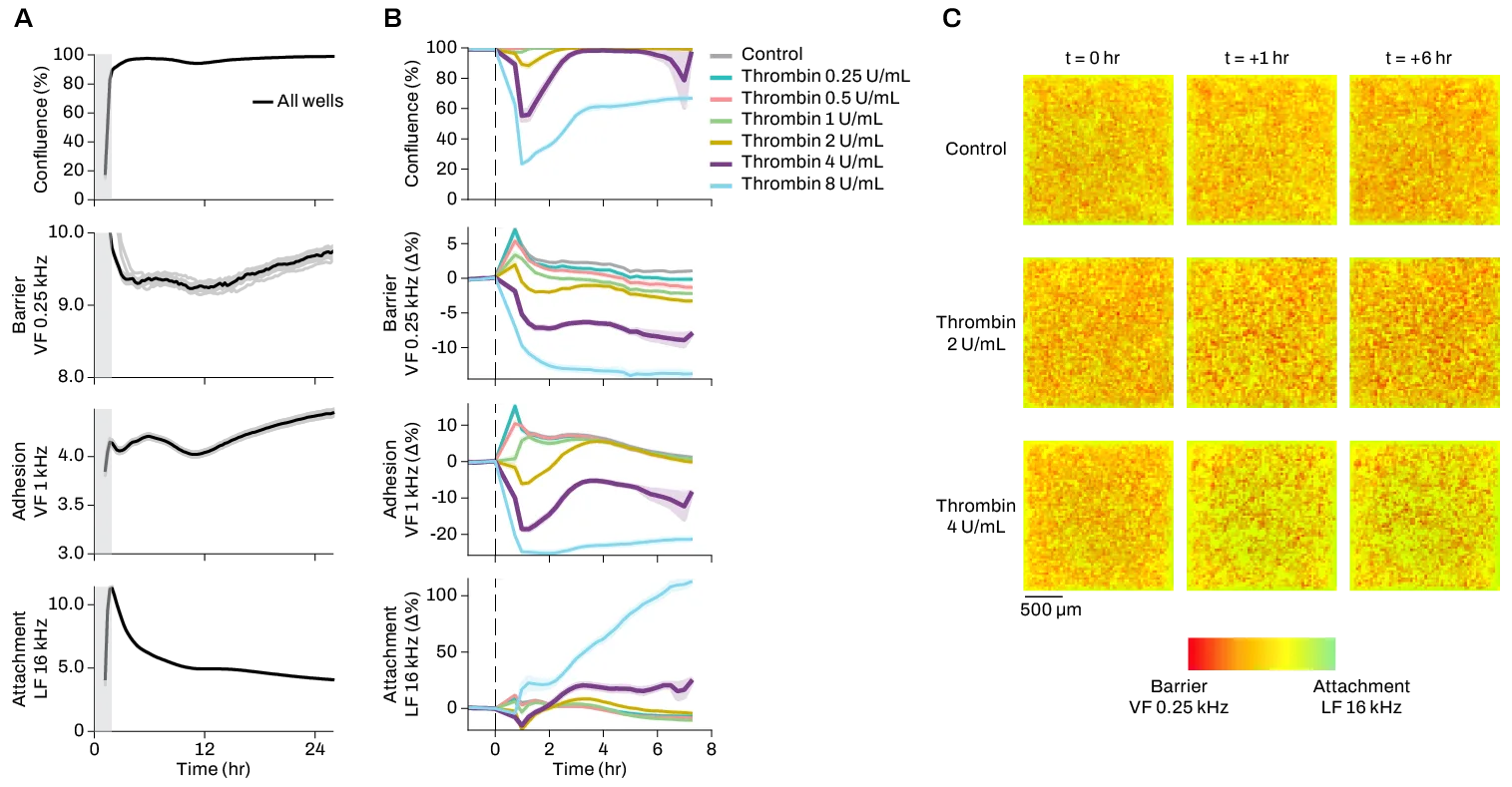
With a uniquely flexible MEA configuration, Pixel is capable of simultaneously taking electrophysiological and electrical impedance measurements in the same well. This allows users to monitor both neural function and phenotypic properties like cell viability, morphology, and motility in the same experiment with 400um resolution for electrophysiology, and 12.5um resolution for electrical imaging. Unlike many other imaging methods, Pixel electrical imaging does not require labels, dyes, or transgenic cells; instead, Pixel uses changes in electrical activity to record multiparametric data on cell health, structure, behavior, and more.
By combining high-resolution cell electrophysiology and phenotype measurements in one experiment, Pixel provides unparalleled insights into neural form and function at multiple scales. Contact our team to learn more about using Pixel for your neuroscience application.