What is metastasis?
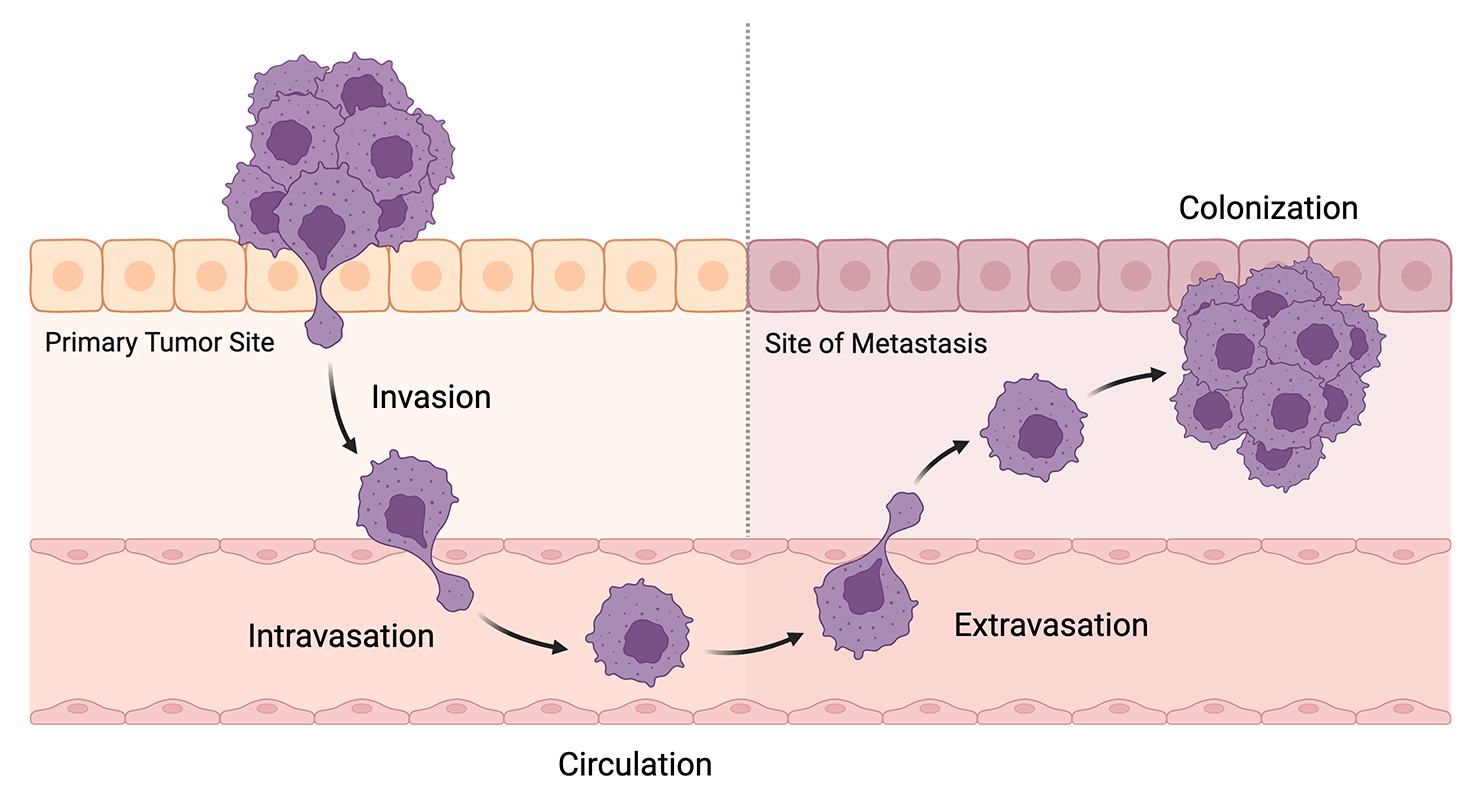
Metastasis refers to the process of cancer cells from a primary tumor spreading to another part of the body. It accounts for more than half of deaths in cancers caused by solid tumors, with estimates reaching as high as 90%. This process broadly happens in several key steps known as the metastatic cascade:
- Invasion: Cancer cells detach from the primary tumor and enter the basal lamina, a layer of extracellular matrix that separates the tumor from connecting tissue and vasculature.
- Intravasation: These detached cells break through the basal lamina and enter the bloodstream.
- Circulation: The cells survive in the bloodstream and travel to other parts of the body.
- Extravasation: Cells once again break through the basal lamina to exit the bloodstream and take up residence in a different tissue.
- Colonization: The cells replicate and develop into a new tumor which resembles the primary tumor.
Schematic of the metastatic cascade. Created with BioRender.
Most cells do not make it all the way through the cascade, instead dying as a result of immune surveillance, inability to adapt to the new microenvironment, or other mechanisms. Only the most resilient cells survive, begetting a new population of tumor cells that are particularly invasive, proliferative, and resistant to cell death.
How do cancer cells become metastatic?
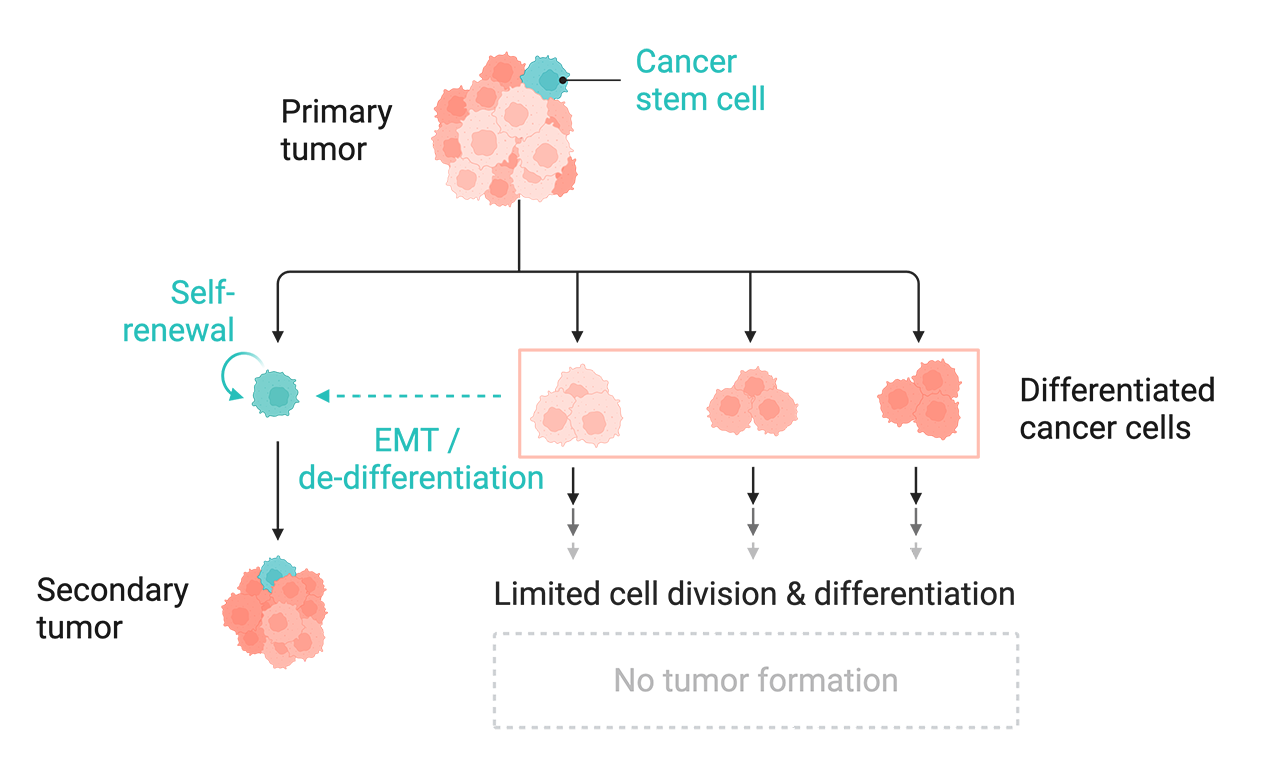
The metastatic cascade is primarily driven by a renewable population of cancer stem cells (CSCs): pluripotent cells that can differentiate into all the other cell types that make up a tumor. CSCs not only exhibit a heightened capacity for self-renewal, but they are also generated by differentiated cancer cells through a de-differentiation process called the epithelial-mesenchymal transition (EMT).
Cells undergoing EMT exhibit a shift towards a mesenchymal phenotype, which includes enhanced motility, reduced cell-cell adhesion, resistance to apoptosis, and stem-like self-renewal properties. These properties give CSCs an enhanced ability to invade, survive, multiply, and seed new tumors in diverse tissue microenvironments. Importantly, EMT-driven lineage plasticity enables this heterogeneous cell population to sustain itself from a constant supply of new CSCs.
Tumors are composed of heterogeneous cell populations. Among them are CSCs, which can renew themselves and form secondary tumors. Differentiated cancer cells can also become stem cell-like via processes like EMT. Created with BioRender.
Why is metastasis associated with treatment resistance?
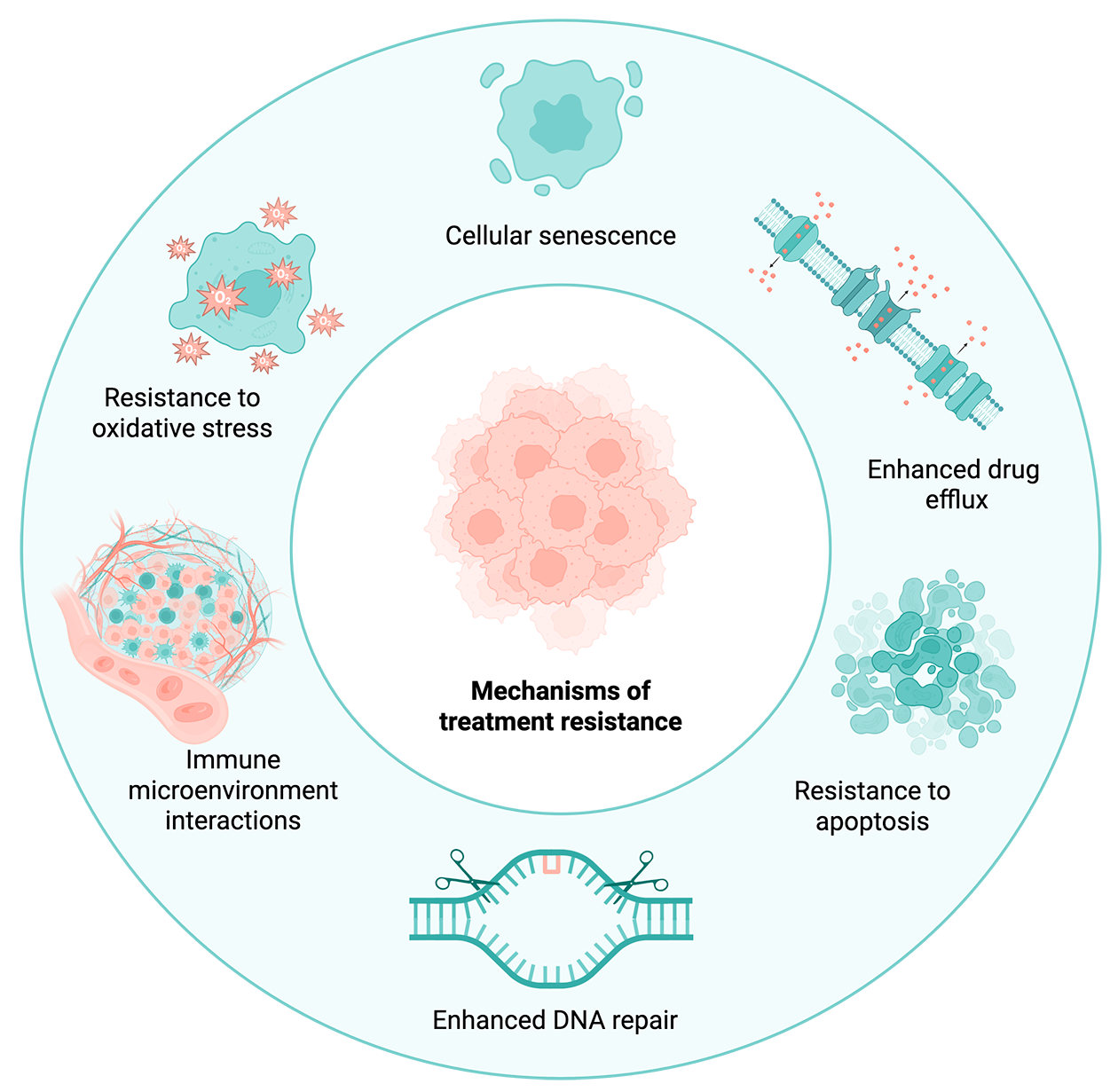
Metastasis acts as a selection process for particularly resilient subpopulations of cancer cells that can invade and thrive in new niches. The characteristics that confer this resilience also help metastatic cells evade drug and radiotherapies. Cancer cells exploit a variety of mechanisms to escape attempts at killing them, including:
- Entering cellular senescence, a reversible state of dormancy where cells stop dividing – hiding them from drugs that target rapidly dividing cells
- Expression of drug efflux pumps, which are transporter proteins that help cells remove toxic material like drug compounds
- Enhanced DNA repair mechanisms that enable cells to fix otherwise lethal DNA damage from drugs and radiation
- Increased expression of anti-apoptotic proteins which keep cells from triggering programmed cell death pathways
- Increased resistance to oxidative stress via antioxidant-producing enzymes
Several avenues that can lead to treatment resistance in metastatic cancer cells. Created with BioRender.
In addition to these inherent properties of metastatic cancer cells, tumors also exist in an immunosuppressive tumor microenvironment (TME) where they interact with other cell types that support their continued survival and proliferation. These cell types – notably tumor-associated macrophages (TAMs) and cancer-associated fibroblasts (CAFs) – promote metastasis by inducing EMT, inhibiting local immune responses, preventing apoptosis, and other mechanisms.
How can we overcome treatment resistance in metastatic cancers?
Because of the distinct factors that allow distant tumors to survive, treatments that work on primary tumors are largely ineffective on metastatic cancers, resulting in a drastically lower 5-year survival rate for metastatic (~31%) compared to localized or regional disease (~83%). Strategies to improve treatment efficacy for metastatic cancers must thus focus on the unique processes that enable these tumors to persist.
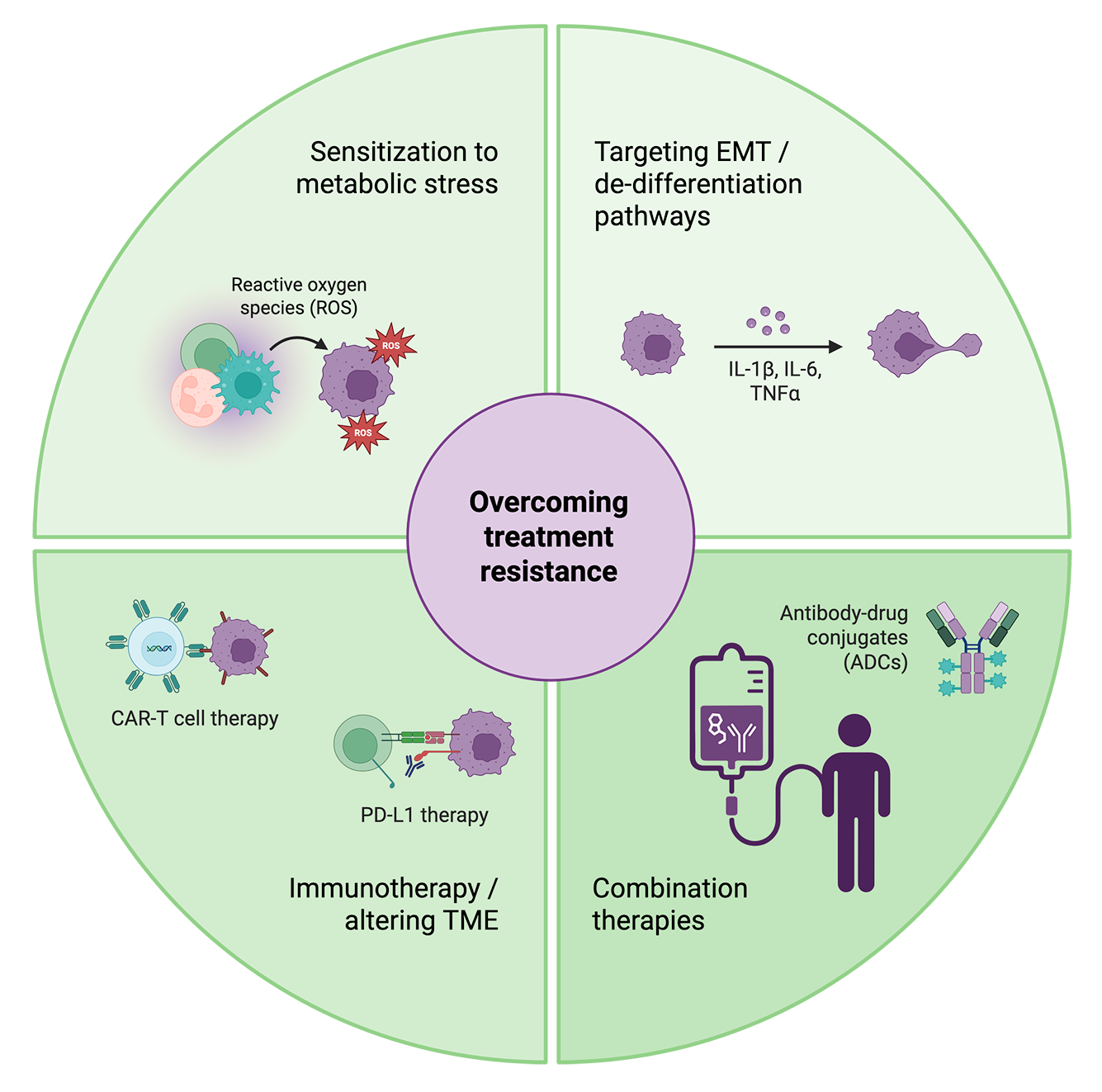
One approach focuses on eliminating tumors’ source of CSCs by targeting pathways involved in EMT, plasticity, and de-differentiation. However, ongoing research reveals that these pathways simultaneously play important roles in tumor suppression and normal physiology, making them difficult to alter without counterproductive off-target impacts.
A potentially less harmful approach is targeting metastasis-specific characteristics that confer fitness and resistance to stress. Metastatic tumors exhibit exceptional metabolic flexibility and oxidative stress; altering pathways involved in fatty acid oxidation and antioxidant production can make them more vulnerable to normal immune responses as well as treatments. These pathways are often lineage-agnostic, or shared between cancer types and tissues of origin, making them broadly applicable across diseases.
A selection of strategies being used in the lab and the clinic to break through treatment resistance in metastatic cancers. Created with BioRender.
Disrupting the tumor’s local immunosuppressive niche, or TME, can also improve treatment response. Immunotherapeutic approaches like targeting immune inhibitory checkpoint proteins and TME-associated cells like TAMs and regulatory T cells (Tregs) have been shown to promote anti-tumor immune response and sensitize tumors to other therapies.
Metastatic cells leverage many adaptations – from structural to environmental – to persist and evade treatment. Targeting multiple adaptations at once by combining therapeutic approaches is proving to be a more effective strategy than monotherapy in treating several types of metastatic cancers.
How do we assess the efficacy and safety of these treatments?
Animal models have traditionally been used in cancer drug evaluation. However, as human cell-based assays improve at recapitulating disease biology, these in vitro systems are increasingly used as a more clinically relevant alternative or complement to animal testing. In vitro models can be cultured directly from patient-derived tumor cells, providing a powerful tool for probing metastatic biology, identifying and validating potential targets, and assessing personalized treatment response at scale. Complex models like 3D organoids and co-cultures of multiple cell types are also emerging to account for the significant influence of tumor-immune dynamics.
What is slowing us down in developing more effective treatments?
Metastasis is a complex process characterized by changes in multiple aspects of cell phenotype, from morphology and extracellular structure to motility and behavior. Capturing this information in vitro typically requires multiple assays, each optimized to measure just one or two parameters and performed on a different set of cells. For more advanced 3D models and heterogeneous cell populations, these assays are even more laborious and time-intensive, often requiring novel methods.
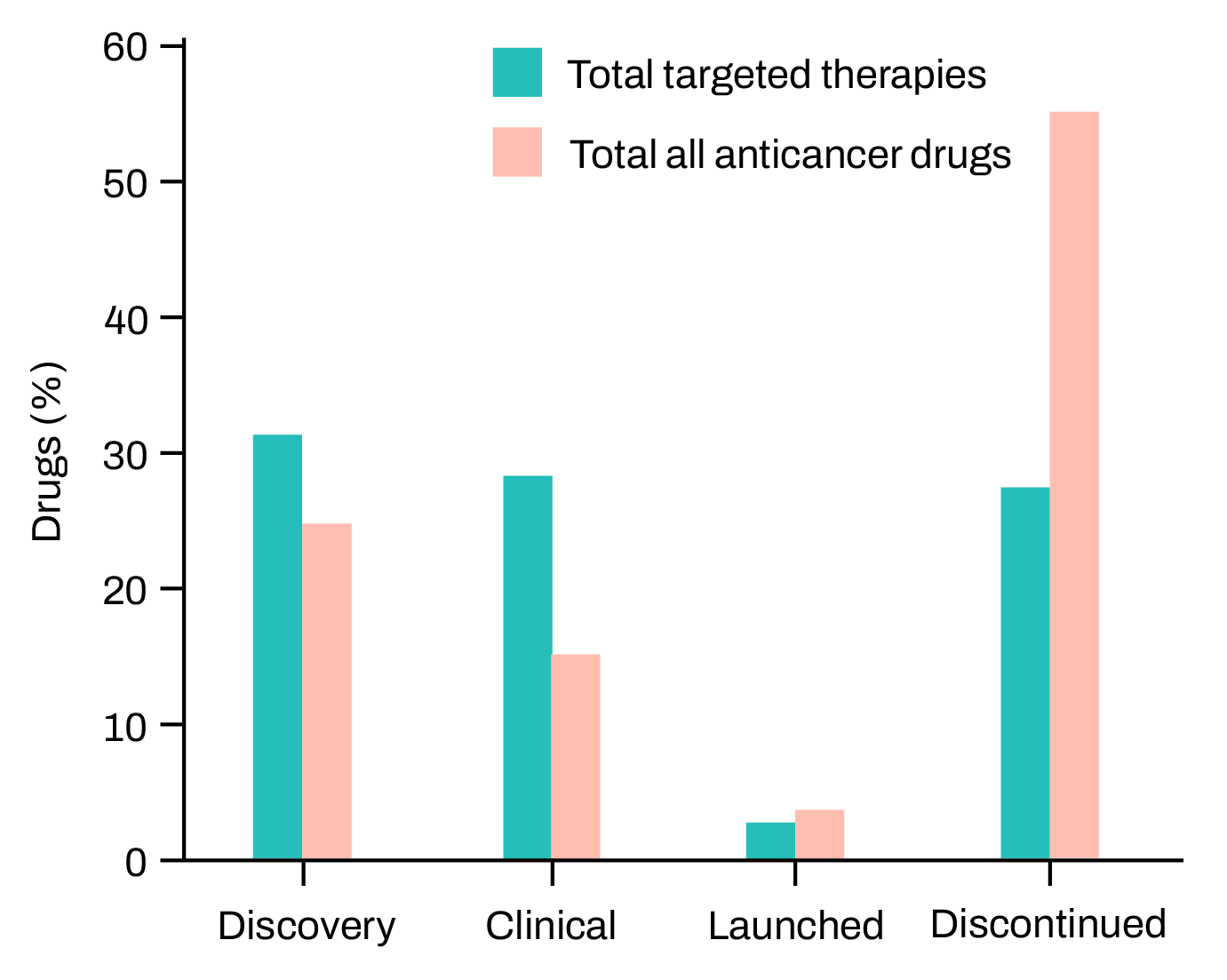
Cancer drugs are much less likely to get regulatory approval than drugs in other therapeutic areas – only 5% that show promise in preclinical studies go on to become approved drugs. Figure adapted from Hutchinson & Kirk (Nature Reviews Clinical Oncology 2011).
In drug screens, most of these cell properties are not captured at all: instead, many assays rely on single end-point readouts measuring cell death in response to a compound. While killing cancer cells is the primary aim, it’s important to assess the full phenotypic response of cells to a drug to determine its mechanism of action (MoA) and potential off-target effects. End-point readouts also lack information on response kinetics – how cells respond to drug treatment over time.
How can we accelerate the development of targeted treatments for metastatic cancers?
Combining patient-derived cells with high-throughput, multi-modal in vitro screening methods is a promising strategy for speeding up the discovery and validation of drug targets and personalized treatments.
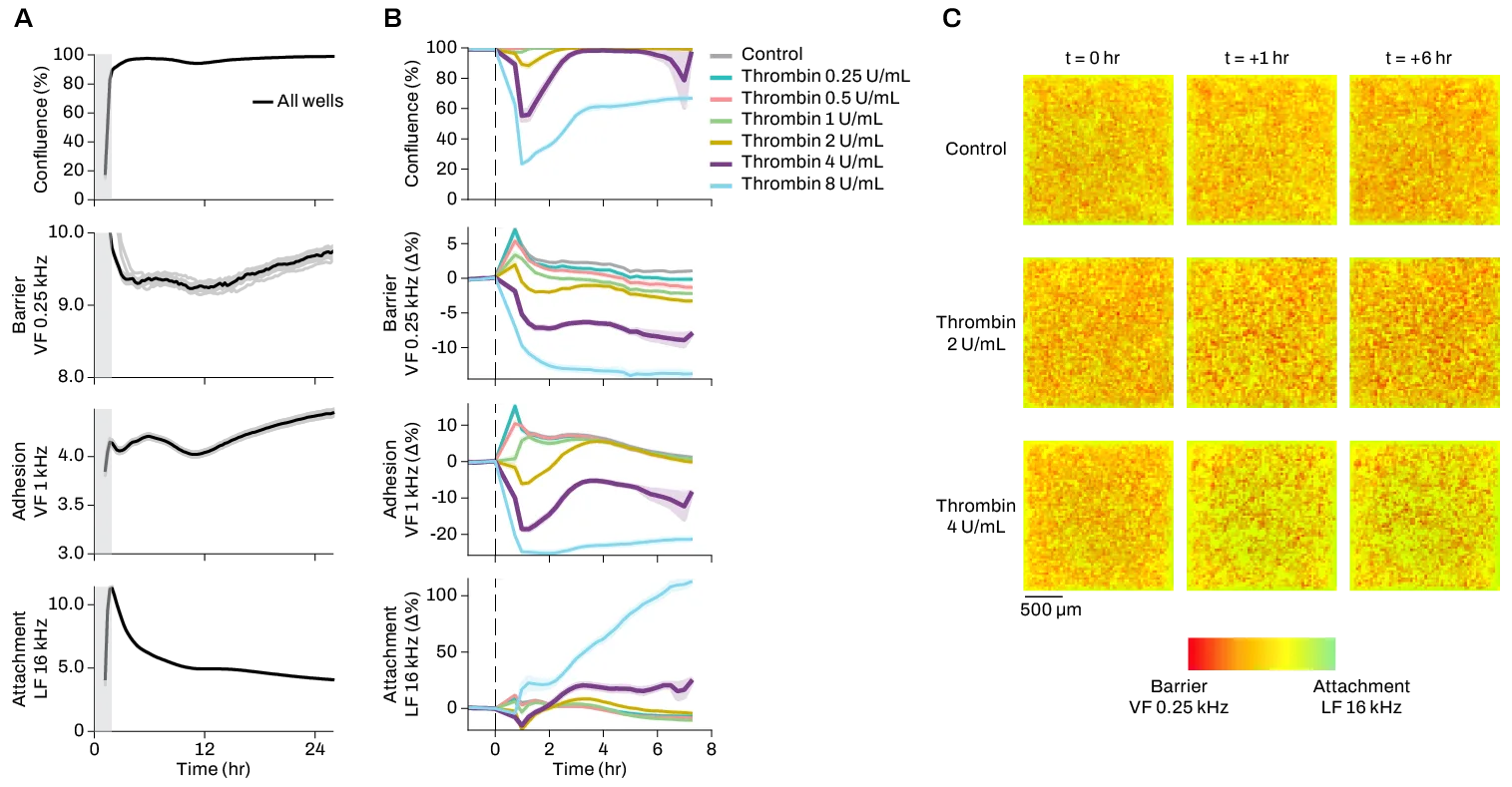
The CytoTronics Pixel™ system reduces multiple assays to just one multiplexed experiment, generating longitudinal, high-dimensional data on 20+ cellular properties for the same set of cells. By capturing complex phenotypic changes over time, Pixel provides live-cell insights on underlying mechanisms of metastasis, as well as response kinetics and MoAs of anti-metastatic treatments. Pixel’s high spatial resolution also enables monitoring of distinct cell populations in co-cultures and complex cell models, which is critical for assessment of TME dynamics and heterogeneous models of tumors.
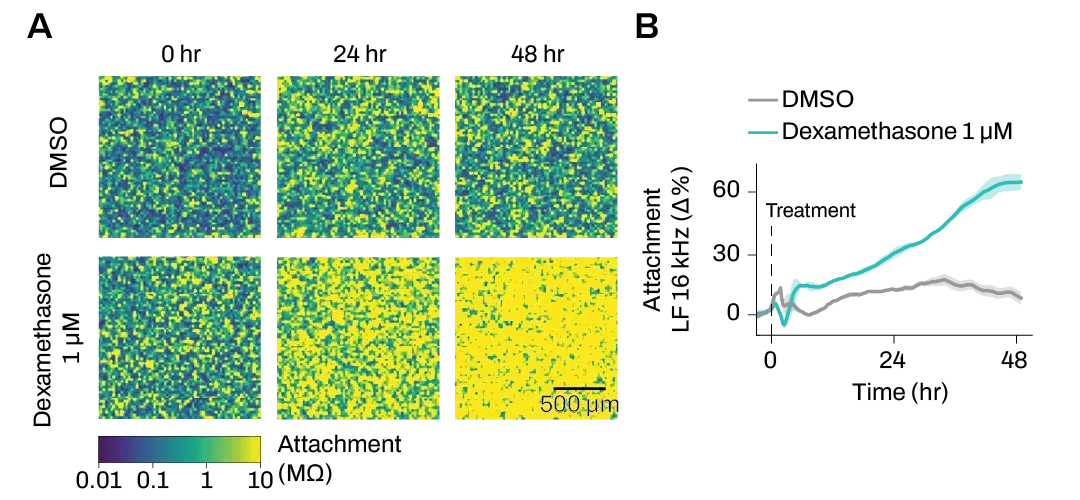
Attachment and motility are two properties that change significantly in metastatic cells. These electrical imaging data show changes in breast cancer cells’ attachment over time after treatment with the anti-metastatic agent Dexamethasone. Click to view effects on cell motility.
Pixel was designed for this pivotal moment in cancer biology. The next generation of therapeutics will be built on increasingly large, multi-dimensional datasets, and scaling insights will be essential to making effective metastasis-targeted treatments a reality.