How does the cardiovascular system work at the cellular level?
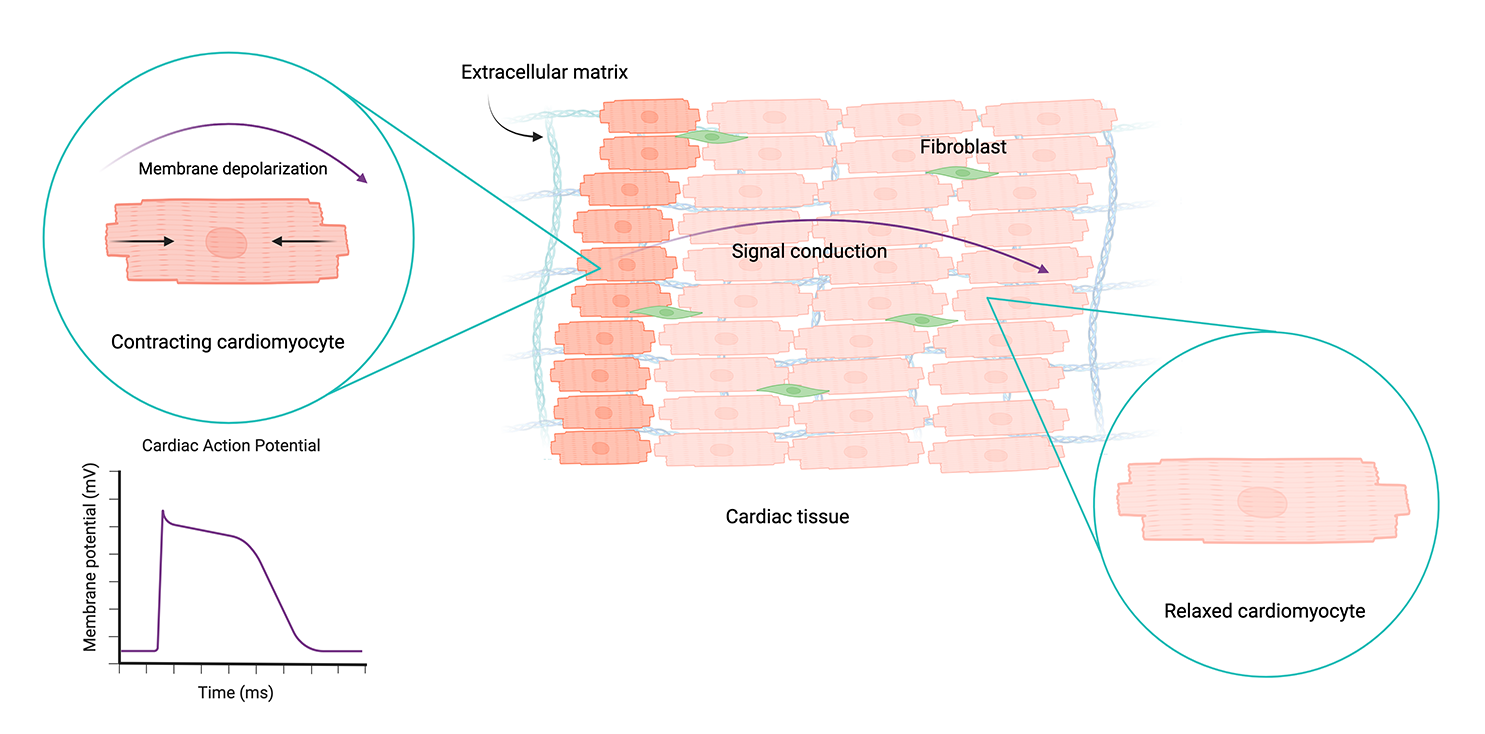
Cardiac tissue is primarily made up of specialized muscle cells called cardiomyocytes, interspersed with other cells called fibroblasts and a collagen-based extracellular matrix. Cardiomyocytes have unique electrophysiological, mechanical, and structural properties that enable them to power the heart and circulatory system.
Mature, healthy cardiomyocytes form interconnected tissue that beats together as a cohesive unit, regulating heart rhythm and rate. This occurs through a paired excitation-contraction process in which electrical impulses, known as action potentials, trigger mechanical contractions of the cells. Action potentials propagate through the tissue across channels called gap junctions.
As positive ions flow into the cell, they increase its membrane potential, resulting in membrane depolarization. Healthy cardiomyocytes have a resting membrane potential of around -90 mV. When this reaches a threshold value of -60 to -70 mV, an action potential is generated, which propagates through the cardiac tissue and triggers contraction. Created with BioRender.
How do cardiomyocytes change in response to disease or other factors?
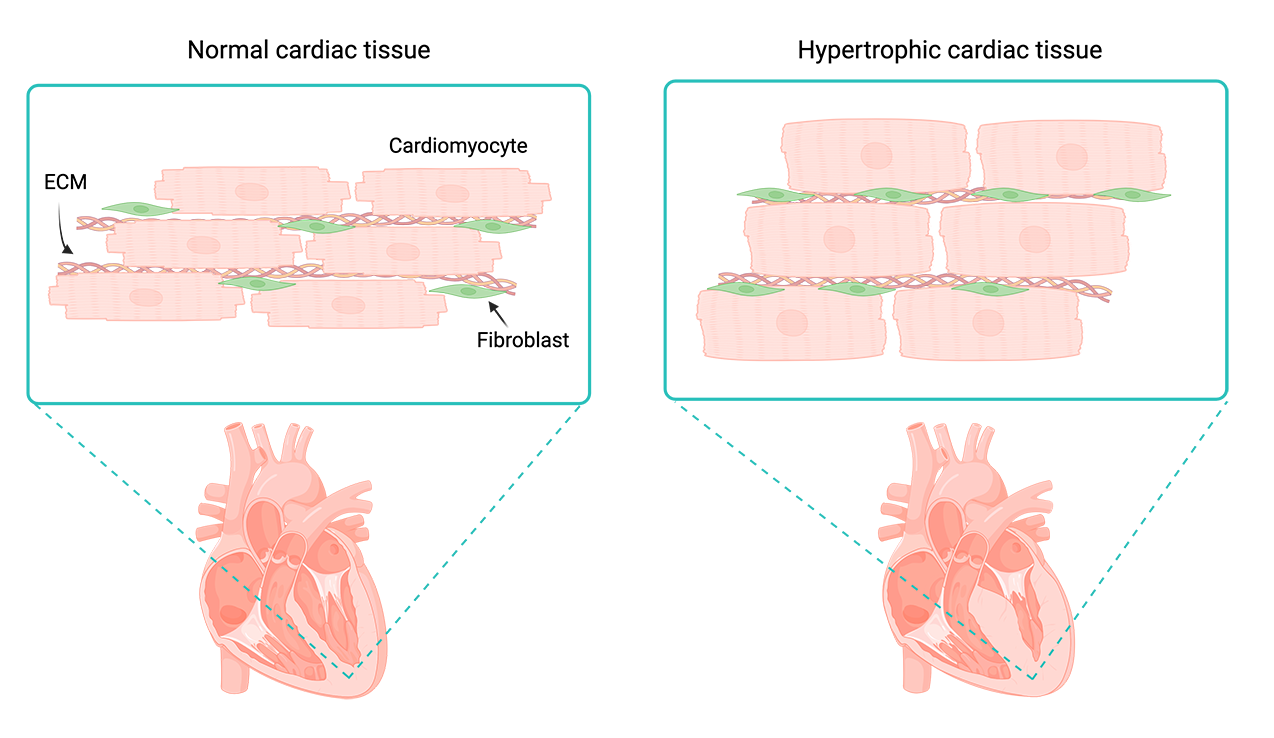
When cardiac tissue experiences an increased workload due to factors like stress, disease, or injury, cardiomyocytes undergo a suite of adaptive changes. Cells may get physically larger, adjust patterns of protein synthesis, and restructure their sarcomeres (muscle fibers) to cope with the added stress: a state called cardiac hypertrophy. Over time, cardiac hypertrophy causes thickening of the heart muscle and impedes cardiac function, potentially leading to cardiovascular complications and heart failure.
Differences in cardiac tissue without hypertrophy (left) and with chronic hypertrophy (right). Prolonged hypertrophy can change cardiac tissue structure and lead to heart failure. Created with BioRender.
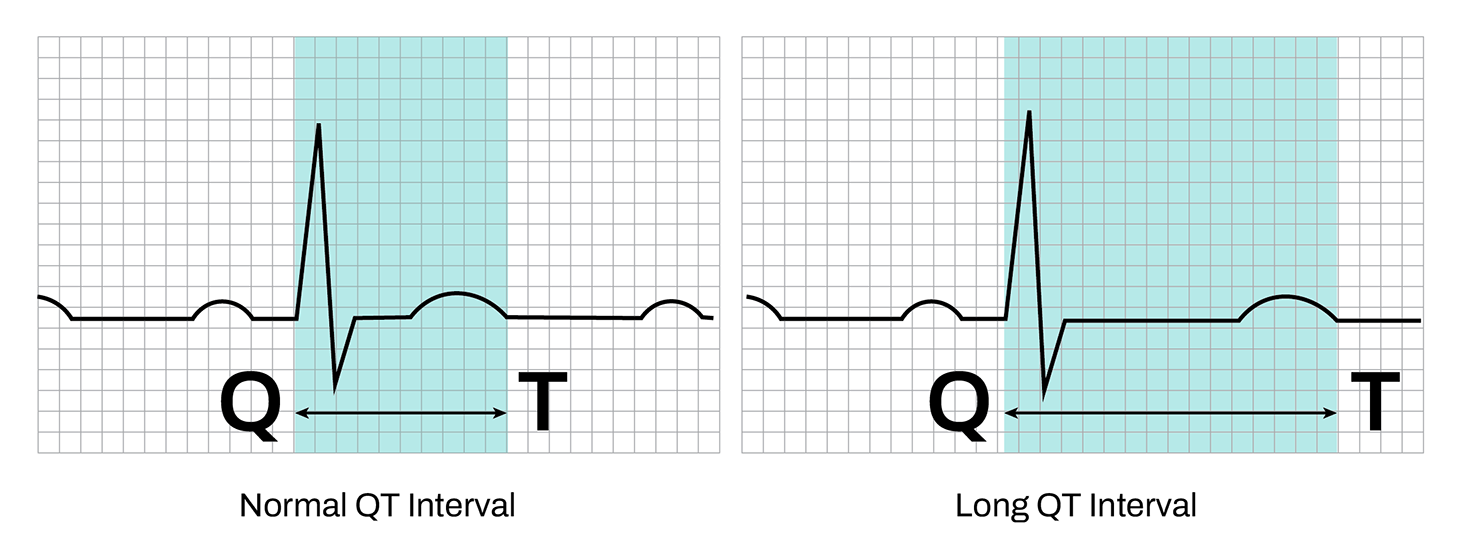
Changes in cardiac tissue can also be chemically induced. Every drug that makes it to market must be tested for negative impacts on heart tissue, or cardiotoxicity. Shortening or prolonging the QT interval – the time between ventricular contraction and recovery in electrophysiological data – is a red flag in cardiotoxicity screens. As changes in QT interval may lead to arrythmia and heart failure, these results can halt clinical trials or even an entire drug pipeline.
QT interval is a measurement of the time between cell contraction (Q) and recovery (T). Abnormally long or short intervals can indicate arrythmias.
What tools are commonly used to study cardiac electrophysiology?
Cardiac electrophysiology is the measurement and study of patterns in cardiomyocyte electrical activity and how they relate to heart function. Most electrophysiology tools fall into two broad categories: imaging-based techniques and direct measurement of electrical currents.
Optical imaging
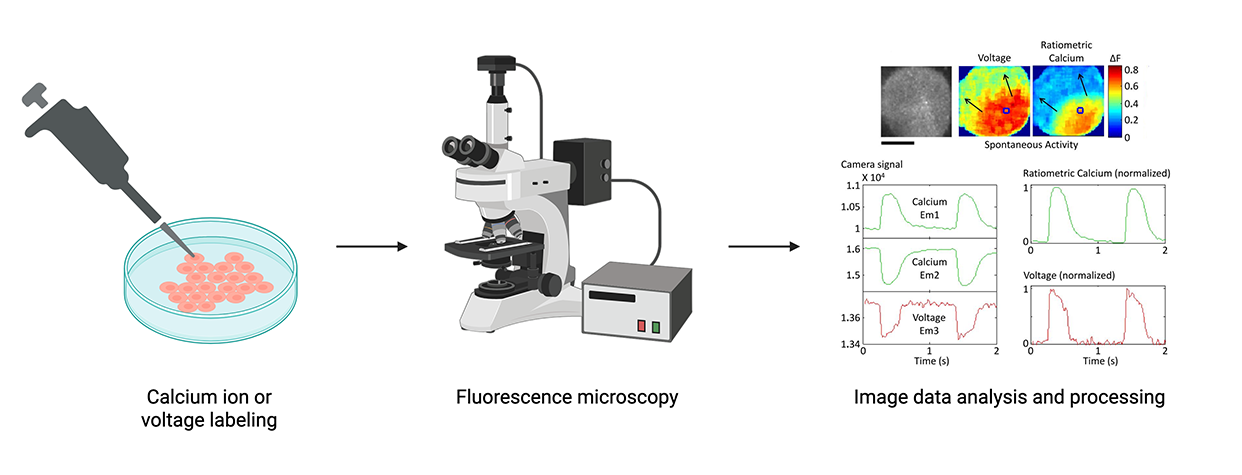
Optical imaging uses microscopy to capture signals produced by fluorescent molecules or proteins. Traditionally, imaging-based methods have used calcium- or voltage-sensitive dyes to enable visualization of electrophysiological activity – a process called optical mapping. More recently, researchers have adopted optogenetic tools to modulate cardiac cell activity using light-sensitive opsin proteins. Innovations combining optical mapping with optogenetic stimulation are giving rise to all-optical methodologies for interrogating cardiac electrophysiology.
Some advantages of these techniques include generating data with high spatial resolution, the increasing capacity for automation, and the ability to modulate and capture electrophysiological activity in one experiment. However, processes for introducing labels, dyes, and opsins into cells can require substantial optimization and be potentially destructive. Additionally, exposure to light can lead to a loss of fluorescence called photobleaching, which further limits the use of optical techniques for longitudinal data capture.
Simplified optical imaging workflow using optical dyes. Optical readouts must be analyzed to infer underlying electrophysiological activity. Figures from “Image data analysis and processing” step from Lee et al. (Circulation Research 2012). Created with BioRender.
Multi-electrode arrays (MEAs)
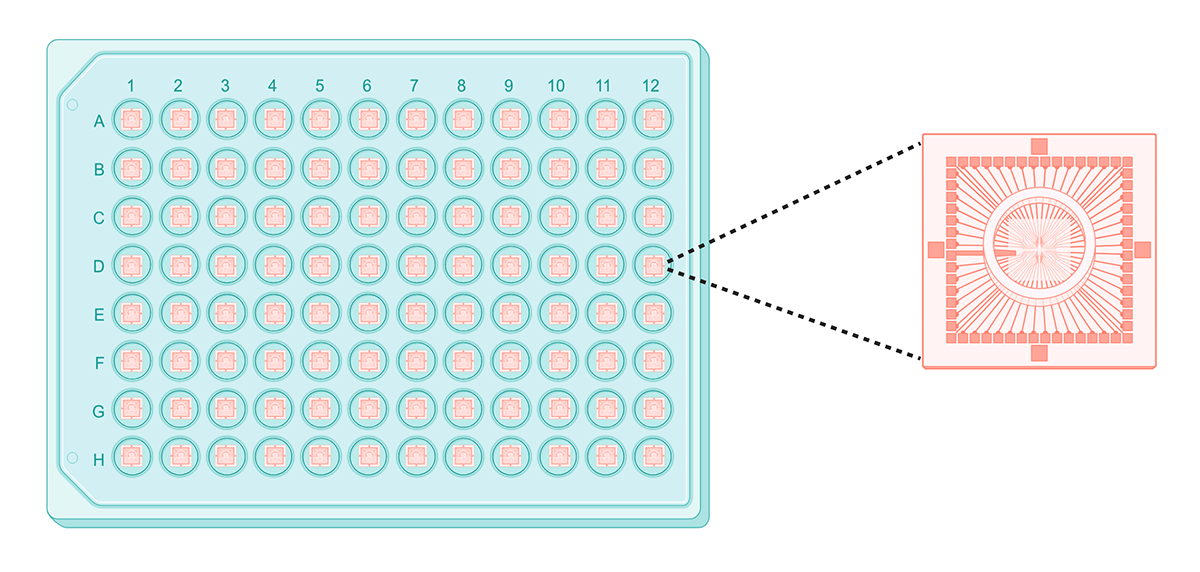
In contrast to optical imaging, electrode-based tools like MEAs directly measure electrical activity in cells. MEAs are multi-well tissue culture plates with electrodes attached to the bottom of each well. The size and density of these electrodes determines the resolution of electrophysiological data, with more tightly-packed electrodes capturing higher resolution data.
MEAs have several advantages over optical imaging-based systems: they don’t require labels or dyes, and can monitor electrical activity in multiple wells of cells (up to 48 or 96) over relatively long periods of time. However, resolution decreases as throughput increases: most MEAs have very few electrodes per well in higher-throughput formats (>24 wells), limiting them to population-level data capture.
A typical multi-electrode array, with a close-up of electrodes in a single well. Created with BioRender.
How do we measure cardiac cell contractility?
Alongside the ability to conduct electrical activity, contractility is another important functional property of cardiomyocytes. Cardiomyocytes’ ability to contract in response to electrical stimulus can be measured with mechanical devices, multi-electrode arrays, or optical imaging-based methods.
Mechanical devices
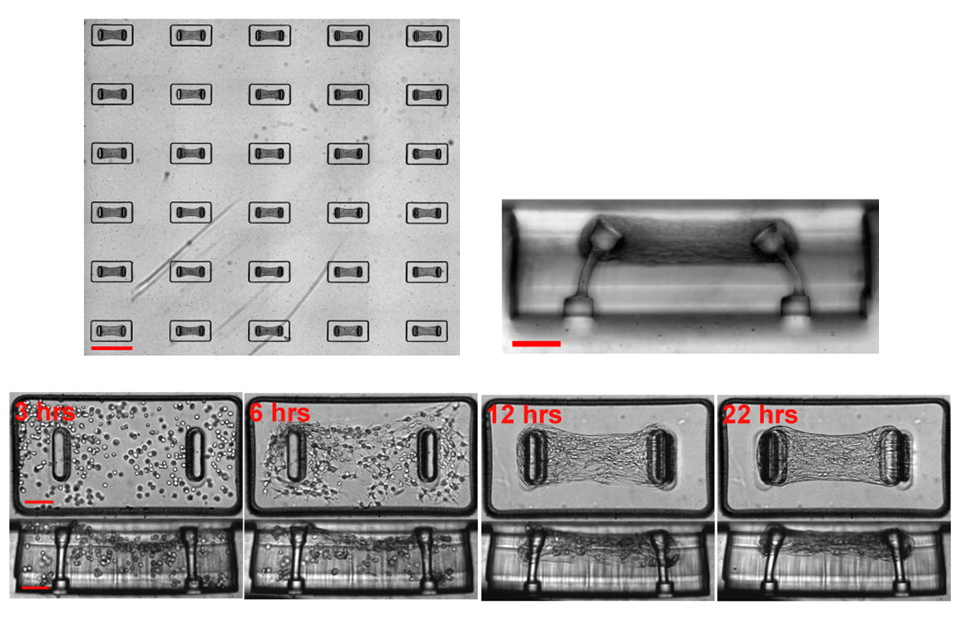
Mechanical methods can capture detailed information at the single-cell level or on 3D samples of cardiac tissue. One technique involves attaching force transducers to opposite ends of a cardiomyocyte or sheet of cardiac tissue and measuring contractile force with a strain gauge or through displacement of an attached needle or fiber. Another method comprises growing 3D engineered heart tissue (EHT) in a specialized platform where the tissue attaches to two microcantilevers that sense and record deflection as cells contract and relax. While these types of experiments can provide high-resolution insights on contractile force, they are difficult to implement and not easily scalable.
Array of micro- cardiac tissues attached to microcantilevers, from Legant et al. (PNAS Engineering 2009). Time course shows microtissue forming between cantilevers, and deflection in cantilevers as microtissue contracts.
Optical imaging
In optical imaging-based methods, high-resolution microscopy is used to image cardiomyocytes at several time points or record videos over several days, gauging contraction by measuring their displacement across the imaging data. These techniques can also generate high-resolution data – and in the case of video recording, longitudinal data – but require complex image processing steps.
Multi-electrode arrays (MEAs)
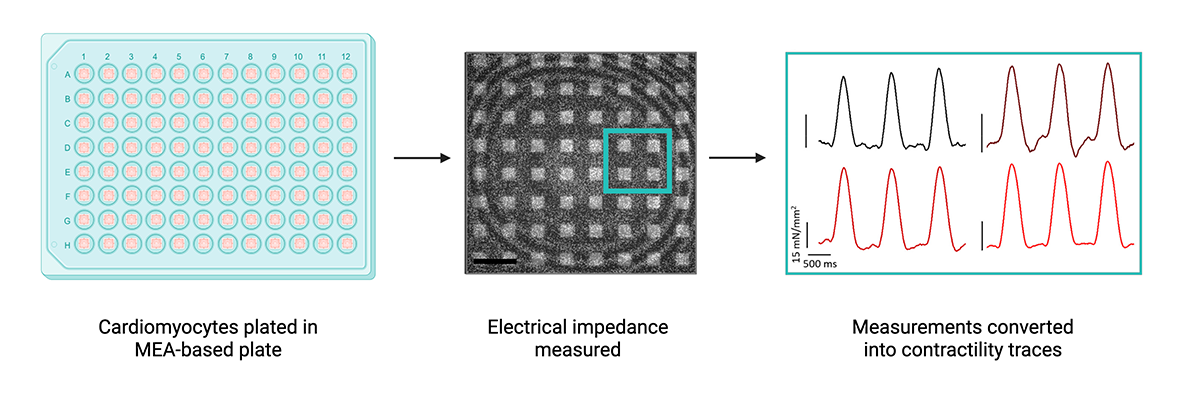
Multi-electrode arrays can be used to assess cell contractility as well as electrophysiology. Unlike electrophysiology applications, electrodes in this case measure cellular impedance, or the perturbation of electrical current, to gauge physical changes in cardiomyocytes that would indicate contraction. However, many MEAs utilize large electrodes to take impedance measurements over a population of cells, providing low-resolution data with relatively high variability. Like mechanical and optical methods, MEAs also only generate a single readout, capturing only one component of cardiac function.
MEAs can measure electrical impedance, which can be used to gauge cardiac cell contraction. Impedance and trace images from Boschi et al. (Nano Letters, 2024). Created with BioRender.
What are some major challenges in studying cardiac function in vitro?
Cardiomyocytes are complex: they conduct electricity, transform electrical impulses into synchronized beating, and confer critical mechanical and structural support to cardiac tissue. Data on all of these parameters are necessary to build a holistic understanding of cardiac function. With the traditional tools described above, this approach requires multiple assays on different sets of cells. Not only does each of these experiments take time and resources (including valuable cells) to set up and optimize, but the resulting data must be normalized across different sets of cells.
As cardiovascular research and drug development shifts away from animal models and towards in vitro systems with more translational relevance, scaling experiments without compromising data quality is a pressing concern. Existing measurement tools generally require a trade-off between throughput, resolution, and number of readouts, prohibiting large-scale, comprehensive studies of cell function and toxicity. Additionally, spatial context is necessary to make sense of how electrical activity propagates throughout cardiac tissue – a dimension that is often missing from high-throughput studies.
How can we overcome these challenges?
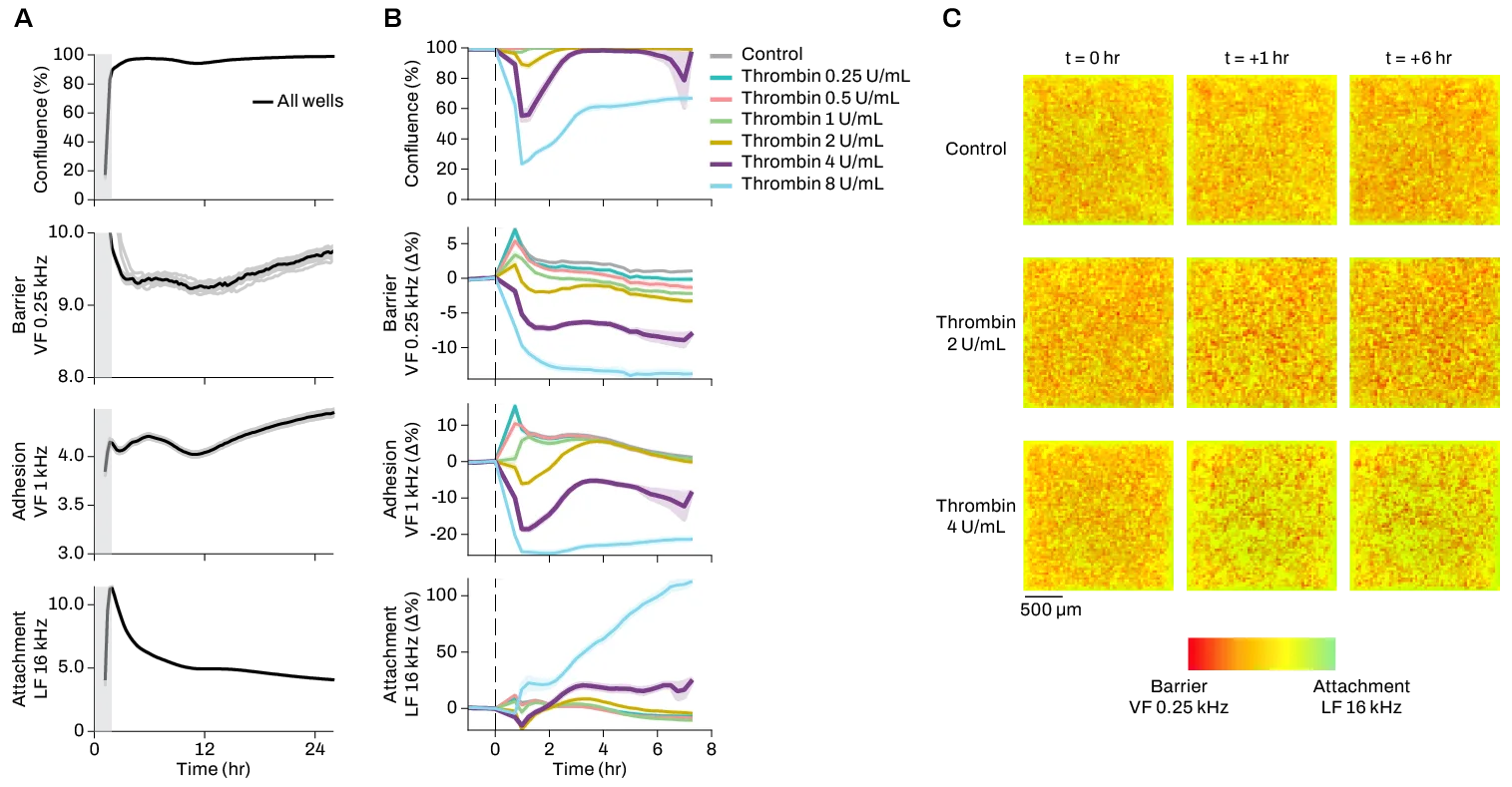
Extracting as much data as possible from the same cell culture not only maximizes precious resources, but also improves data relevance and experimental reproducibility. The Pixel system is the only in vitro live-cell monitoring system that can measure electrical activity, contractility, and signal conduction across a culture at the same time.
Pixel captures high-resolution contractility data with up to >64 recording channels in each well of a 96- or 384-well microplate.
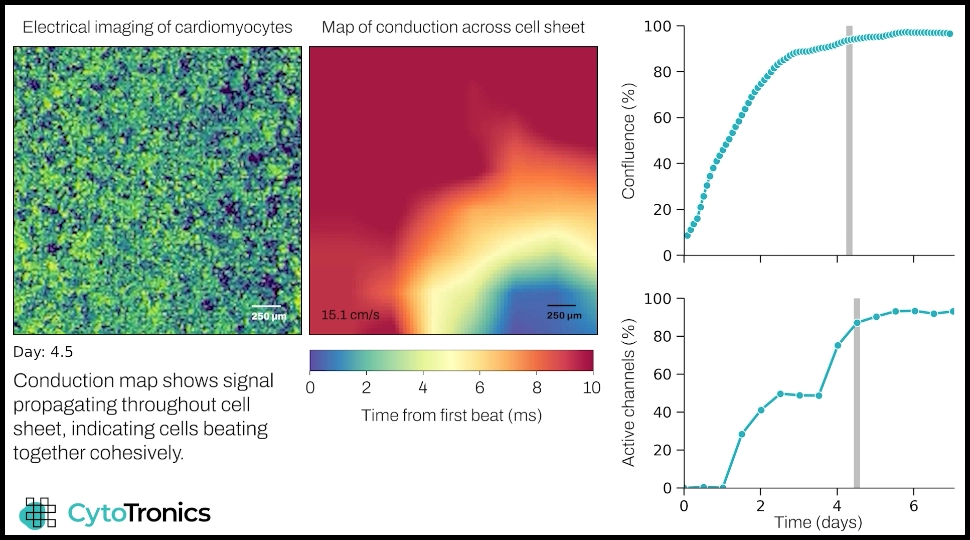
In addition to this comprehensive picture of electrophysiological and mechanical function, Pixel simultaneously takes impedance measurements that provide detailed electrical imaging data on structural and morphological changes in individual cardiomyocytes and cardiac tissue. The video below shows maturation of a cardiomyocyte cell sheet, with culture dynamics visible in the electrical imaging video on the left, and propagation of electrical signal throughout the cells visible in the conduction heat map.
Pixel captures multimodal data from cardiac cells. The conduction map shows how cells initially beat in isolated clumps, while after a few days the signal traverses the culture, indicating the development of a functionally cohesive cell sheet. The plots show culture growth (top) and percentage of beating cells (bottom).
From cardiovascular disease characterization to large-scale cardiotoxicity screening on both 2D and 3D models of cardiac tissue, Pixel offers rich insights for a variety of cardiovascular application areas. Microchip-based technology is built into standard 96- and 384-well microplates that can be read from 1 to 8 plates at a time, and are compatible with both monolayer cell cultures and 3D models like spheroids. Contact our team to learn more about using Pixel for your studies.