What is the blood-brain barrier?
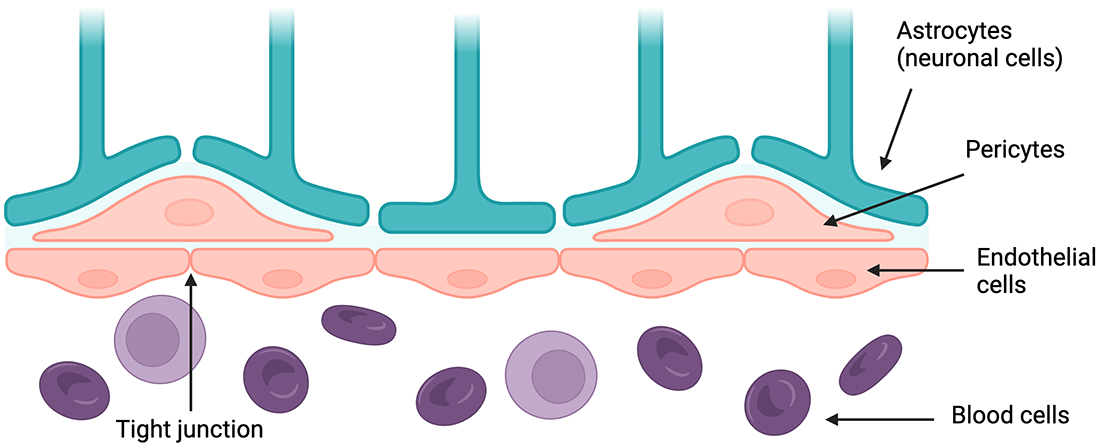
The blood-brain barrier (BBB) is a term that describes the endothelial cells lining the blood vessels of the central nervous system (CNS). In combination with contractile cells called pericytes and neuronal cells called astrocytes, these endothelial cells possess unique properties that strictly regulate vascular permeability. Tight junctions between closely packed endothelial cells limit exchange of materials between the bloodstream and the CNS. The BBB maintains the unique microenvironment necessary for healthy CNS function by both preventing harmful blood-borne materials from entering it and facilitating exchange of key biomaterials like ions and nutrients.
Diagram of key parts of the BBB. Created with BioRender
Why is it crucial to maintain the integrity of the BBB?
What can damage the BBB?
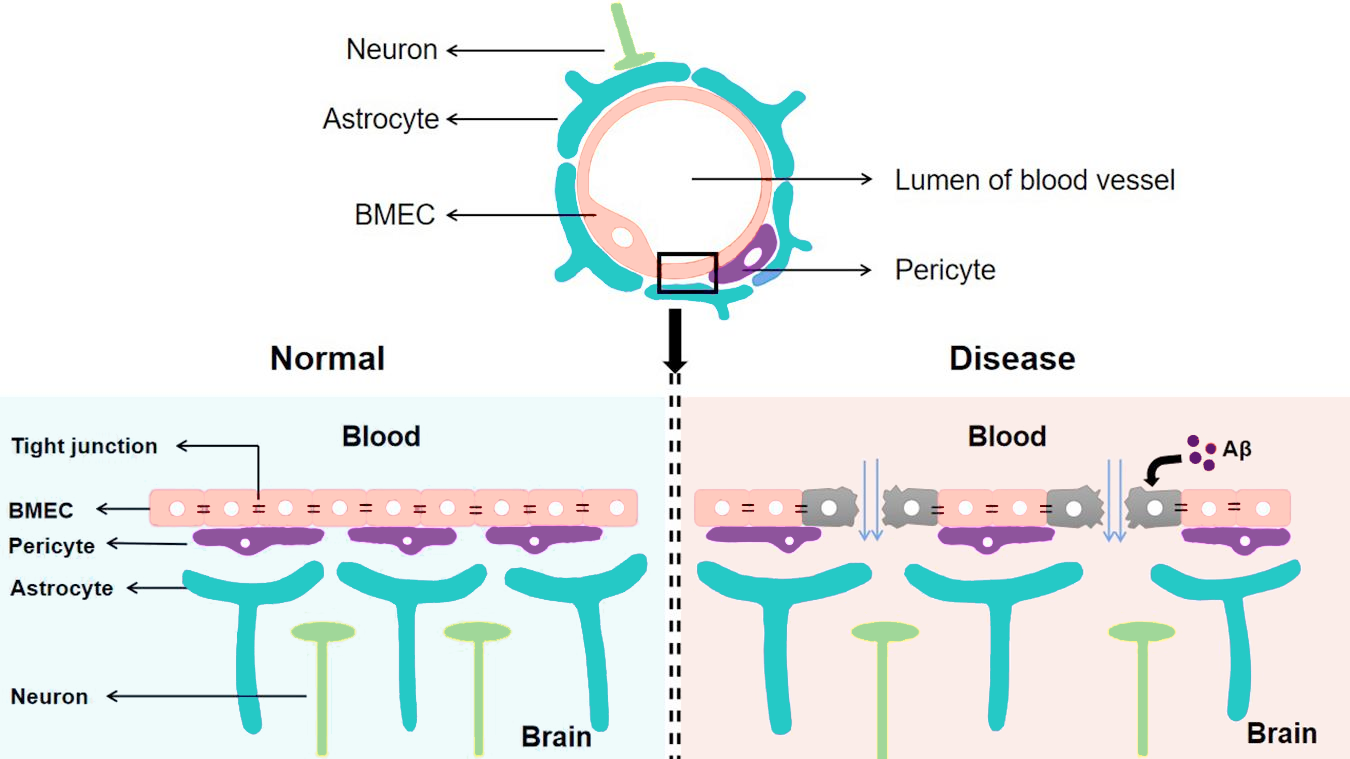
As the BBB plays a critical role in regulating nervous system homeostasis, even minor, transient deviations in its structure and behavior can lead to severe neurological pathologies, like those that characterize Alzheimer’s and Parkinson’s diseases. Weakening of tight junctions, degeneration of endothelial cells and pericytes, impaired cellular transport mechanisms, and other changes can all contribute to increased BBB permeability, allowing harmful biomaterials and their intermediates to accumulate in the brain.
Alzheimer’s and Parkinson’s diseases are both associated with the accumulation of aberrant protein deposits in the brain: amyloid-beta aggregates in Alzheimer’s and Lewy bodies composed of alpha-synuclein in Parkinson’s. Disruption in interplay between these proteins and the BBB results in decreased clearance and accumulation of protein aggregates in the brain.
The BBB in a healthy brain vs. a brain with Alzheimer’s disease. Modified from Wang et al. (Front. Cell. Neurosci. 2021).
There are many factors that can contribute to BBB breakdown, including aging processes, genetics, and traumatic injury. In normal aging, increased oxidative stress can impair the function of endothelial cells, pericytes, and BBB-associated immune cells in a variety of ways, such as upregulating inflammatory cytokines and chemokines and producing harmful reactive oxygen species. Aged endothelial cells also exhibit reduced mitochondrial function and regenerative capacity, further impairing barrier function.
Genetic mutations put some individuals at higher risk of BBB-related dysfunction than others. For example, the APOE4 gene, which encodes Apolipoprotein E4, has been found to accelerate breakdown of the BBB via a cyclophilin A pathway. APOE4 is highly associated with an increased risk of developing Alzheimer’s disease.
Traumatic brain injury can damage the BBB by directly injuring endothelial cells, impairing blood flow, and disrupting tight junction proteins, all of which lead to increased barrier permeability. Barrier deterioration then triggers neuroinflammation and can lead to downstream issues like blood clots and ischemia.
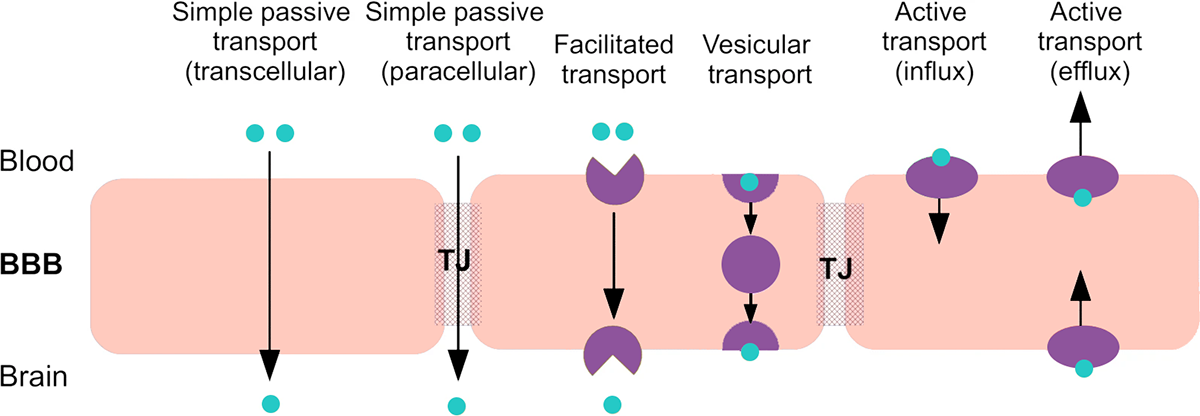
Methods of transport across the BBB. Modified from Vendel et al. (Fluids and Barriers of the CNS 2019).
What tools and in vitro models are commonly used to study the BBB?
Many of the systemic effects of BBB disruption have been garnered from studying in vivo animal models. However, in vitro models provide a closer look at the cellular and molecular-level changes in BBB structure and function that underlie these effects.
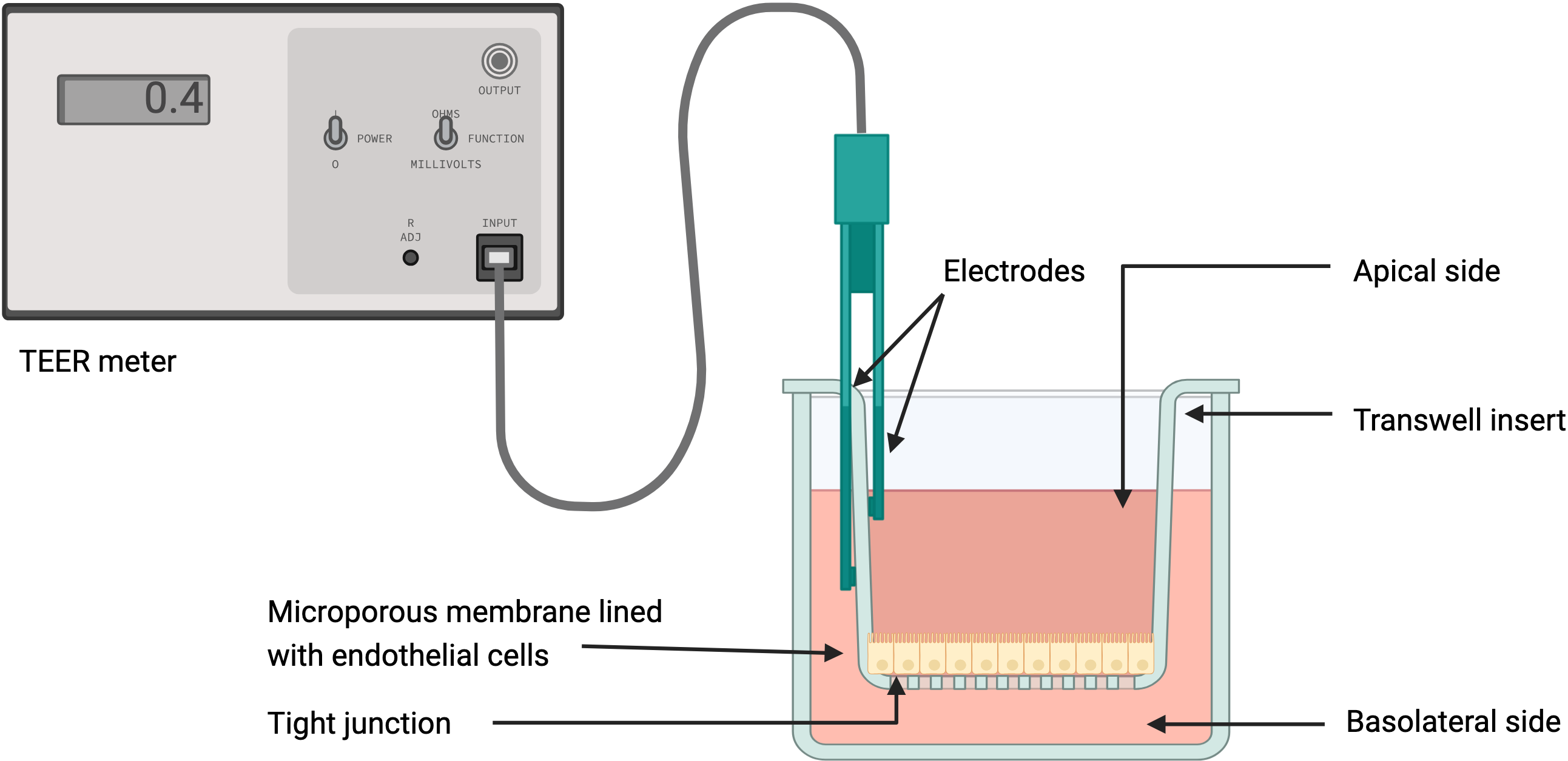
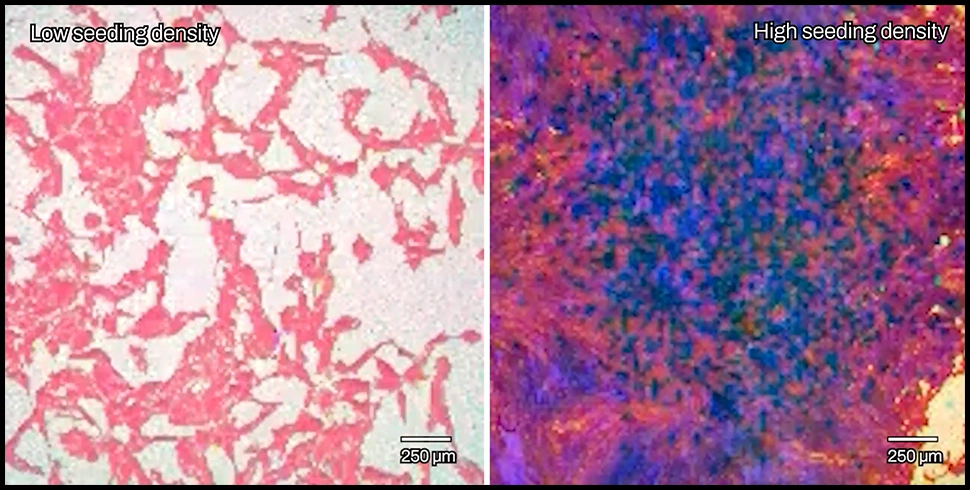
Most in vitro studies of the BBB are performed on a Transwell plate, which consists of an insert with a permeable membrane on the bottom placed into a well full of media. The insert separates the well into two compartments partitioned by the membrane, which is seeded with endothelial cells to mimic the lining of the BBB. The two compartments represent the bloodstream on one side, and the CNS on the other. Perturbations in the system can then be introduced to test changes in barrier function and exchange of materials. For example, different types of cells can be co-cultured on either side of the membrane, or chemical compounds and proteins can be introduced to study corresponding changes in the barrier or compartments.
Diagram of a TEER assay in a Transwell system. Created with BioRender.
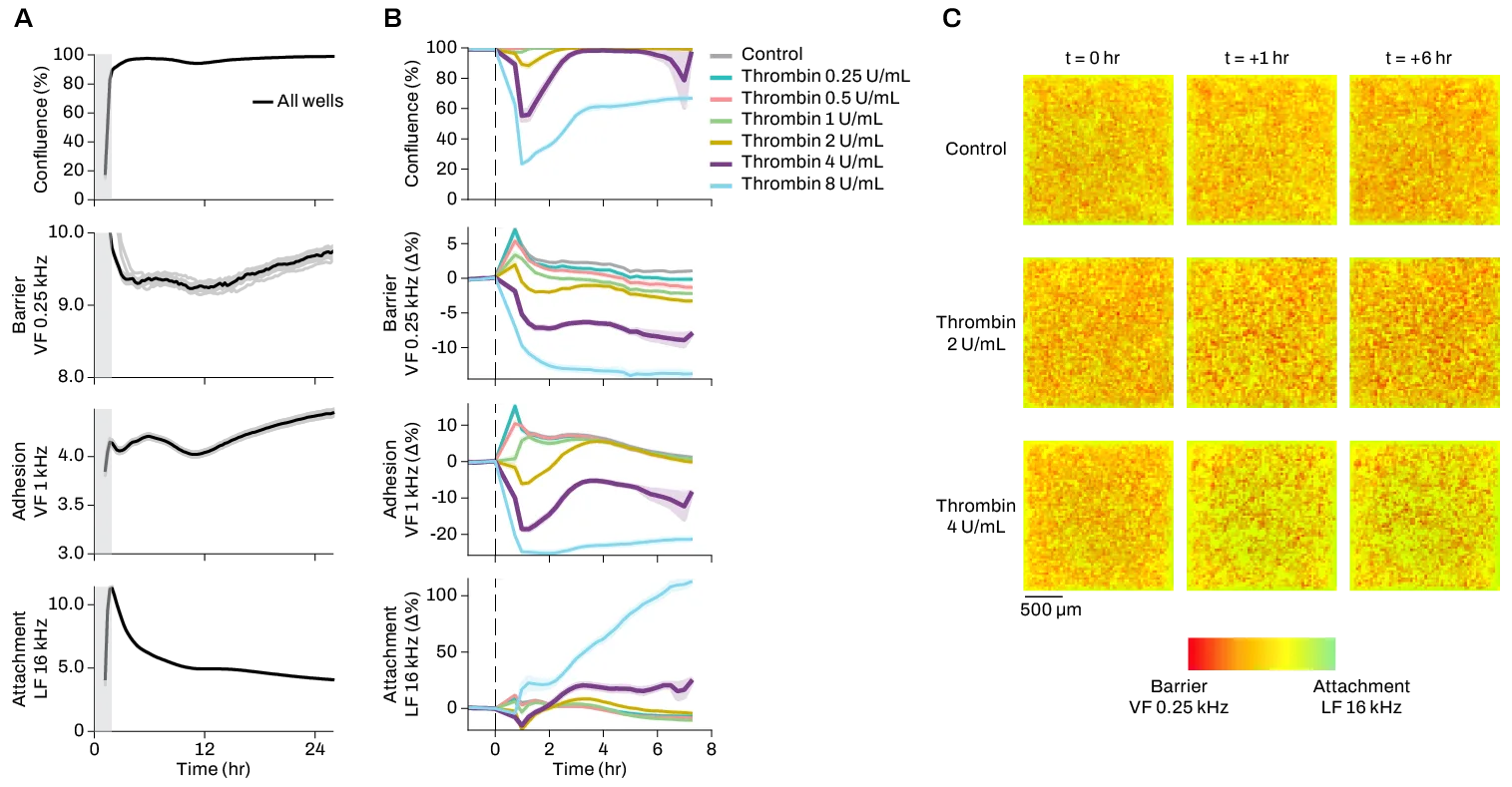
To observe and quantify these changes in barrier behavior and morphology, electrodes are placed in the bottom and top compartments to deliver electrical currents. Patterns of current obstruction, known as impedance, help capture changes in the presence, structure, and behavior of barrier cells. Measuring transepithelial/transendothelial electrical resistance (TEER) can help quantify the behavior of tight junctions and assess barrier integrity.
3D models like those employing organoid and organ-on-a-chip (OOAC) technology are largely derived from this Transwell setup, with various modifications to better mimic the BBB’s physiological context. Some of these models have the added benefit of being able to simulate shear stress generated from the flow of blood, which can help in maturation of the endothelial barrier.
What are the limitations of current tools to study the BBB?
Many tools for studying in vitro models of the BBB are limited by resolution, throughput, and the data types that can be collected during the experiment. First, systems may consist of several electrodes manipulating and measuring electrical activity over many wells, producing low-resolution data by averaging these outputs over large numbers of cells. At these resolutions, deciphering critical cellular-level changes is impossible.
Second, more sophisticated systems that are able to capture more nuanced information are difficult to setup and scale for high-throughput applications. Obtaining higher resolution data requires much more than several electrodes per well, which can be prohibitively expensive and technically complex to set up and optimize even for one experiment.
Third, capturing information on different parameters of cell behavior – such as growth, motility, morphology, adhesion, etc. – requires multiple tools and experiments beyond a single TEER assay, which further limits throughput and replicability. Orchestrating experiments across different platforms, all of which give different readouts, can be tedious and time-consuming. Yet sufficient and accurate multiparametric data are crucial to reveal insights that may be missed by looking at only one or two parameters.
Confluence, impedance, and tissue barrier data from standard large electrode and Transwell system (orange) vs. Pixel semiconductor-based system (blue). Created with BioRender.
How can we improve these tools?
As even miniscule, fleeting changes in the BBB can have far-reaching effects on neural function, capturing these fluctuations in real-time with high sensitivity, throughput, and resolution is essential to gaining translationally relevant insights on the BBB’s role in health and disease. Many existing technologies for in vitro cell modeling and analysis either provide limited information (e.g. live or dead cells) for many samples, or more nuanced outputs (e.g. barrier function) for very few samples. Obtaining data on several cell properties also requires using multiple tools and integrating different data types.
The Pixel™ electrical imaging platform was designed to overcome these limitations: with thousands of electrodes per well, each Pixel plate reader is capable of simultaneously measuring live cells at high resolution across 20+ parameters in real time. Pixel monitors much more than just endothelial cell growth and death, enabling visualization of minute changes in behavior and structure, cell-cell interactions, and barrier formation with unmatched clarity. Pixel also allows you to scale up your assays far beyond existing technologies, with Primo and Octo instruments that can read 96- or 384-well plates.
Learn more in this case study on modeling vascular biology with human umbilical vein endothelial cells, or this application note on modulating the tissue barrier formed by primary human coronary artery endothelial cells.